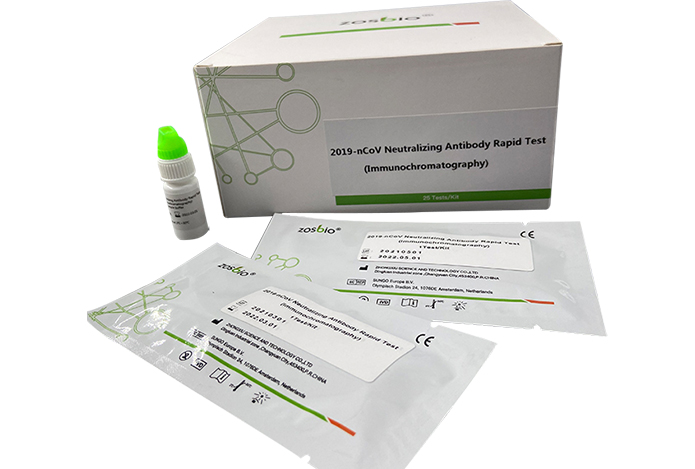


Main Components: test card, a sample buffer (drop bottle), a sample extraction tube, and a drop head.
Wide range of applications: suitable for hospitals, disease control centers, communities,airports, stations, customs, schools, enterprises, etc.
The kit is used for qualitative detection of novel coronavirus (2019-nCoV) neutralizing antibody in human serum, plasma and whole blood samples.
Novel coronavirus (2019-nCoV) is a new coronavirus belonging to β genus. It can cause viral pneumonia, with fever, fatigue and dry cough as the main clinical manifestations, and a few patients with nasal congestion, runny nose, sore throat and diarrhea. In severe cases, dyspnea and/or hypoxemia occurred one week later. In severe cases, acute respiratory distress syndrome, septic shock, metabolic acidosis which is difficult to correct, bleeding and coagulation dysfunction, etc.
Novel coronavirus has several structural proteins, including spikes (S), envelopes (E), membranes (M) and nucleocapsids (N). The spike protein contains a receptor binding domain (RBD), which is responsible for recognizing the cell surface receptor angiotensin converting enzyme 2(ACE2). It is found that the RBD of 2019-nCoV spike protein interacts strongly with human ACE2 receptor, which leads to endocytosis of host cells in lung and virus replication.
After 2019-nCoV infection or vaccination, it will trigger an immune response and produce antibodies in the blood. Secreted antibodies can prevent virus infection, they will exist in the infected human circulatory system for several months to several years, and they will quickly and firmly combine with pathogens, thus preventing virus replication. These antibodies are called neutralizing antibodies. The detection of neutralizing antibody can judge whether people have the ability to prevent virus infection.
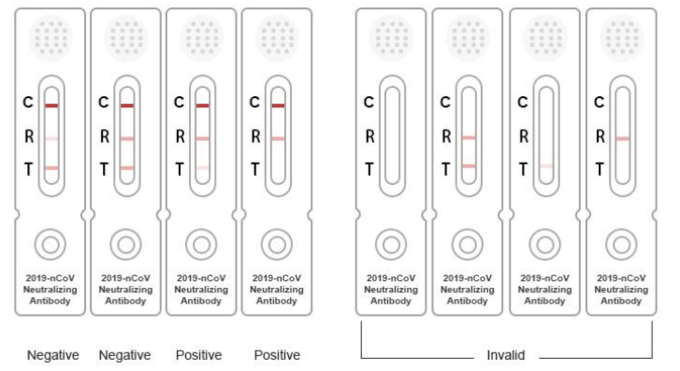
The kit adopts immunochromatography. The test card includes quality control line C, test line T and control line R. The sample to be tested (serum/plasma/whole blood) diffuses upward by capillary action at the loading end. The S-RBD containing the marker in the binding pad binds with the ACE2 protein immobilized on the NC membrane, and the signal can be detected at the position of T line. If there is neutralizing antibody in the sample, it will combine with the labeled S-RBD antigen when it flows through the labeled pad. Neutralizing antibody can prevent the combination of S-RBD and ACE2, thus causing the signal value to decrease. The signal value of T-line is negatively correlated with the content of neutralizing antibody. When the concentration of neutralizing antibody is high enough, T-line will not develop color. No matter whether the T line is colored or not, the control line R and the quality control line C should be colored. The quality control line C is used for quality control. If the color of the line C is not developed, it means that the test is invalid, and the sample must be retested.
The kit consists of a detection card and a sample buffer solution.
Test card: It consists of aluminum foil bag, desiccant, test paper strip and plastic card. The test strip consists of absorbent paper, nitrocellulose membrane, sample pad, bonding pad and rubber plate. T line (detection line) of nitrocellulose membrane is coated with ACE2 protein, C line (quality control line) is coated with quality control line antibody, R line (control line) is coated with control antibody, and the binding pad contains 2019-nCOV antigen labeled by marker.
Sample buffer: phosphate, sodium azide, etc.
Storage Conditions and Validity
Keep it at 2℃ ~ 30℃ for 12 months.
The validity period of the aluminum foil bag is 1h after unpacking.
Batch number of products: see label for details.
Expiry date of products: see label for details.
Sample Requirements
1. Collect serum, plasma or whole blood samples.
2. Sediments and suspended solids in the samples may affect the experimental results and should be removed by centrifugation.
3. Anticoagulants: The use of heparin, EDTA and sodium citrate anticoagulants has no significant effect.
4. Blood collection should be carried out by professional medical personnel. It is recommended to give priority to serum/plasma detection. In emergency or special circumstances, whole blood samples of patients can also be used for rapid detection.
5. Serum and plasma samples should not be stored at room temperature for more than 8 hours, and can be stored for 5 days at 2℃ ~ 8℃, and for 6 months below -20℃, but avoid repeated freezing and thawing. Whole blood samples shall not be frozen and stored at 2℃ ~ 8℃ for no more than 48 hours.
Test Method
Read the instruction manual carefully before testing. Please restore all reagents to room temperature before testing, and the testing should be carried out at room temperature.
1. Take out the test card from the packaged reagent bag and use it within 1 hour.
2. Drop 20μL sample (serum, plasma or whole blood) into the sample adding hole of the test card, then drop 2 drops (about 60μL) of sample buffer, and start timing.
3. Read the results when reacting for 10 ~ 15 minutes at room temperature. The reading result is invalid after 20 minutes.
1. Analysis of specificity
1.1 Cross-reaction: The interference of the following types of antibodies was evaluated with reagents, and the results showed that there was no cross-reaction.
| SN | Item | Cross reaction | SN | Item | Cross reaction |
| 1 | Endemic human coronavirus OC43 | No | 10 | Enterovirus | No |
| 2 | Endemic human coronavirus HKUI | No | 11 | EB virus | No |
| 3 | Endemic human coronavirus NL63 | No | 12 | Measles virus | No |
| 4 | Endemic human coronavirus 229E | No | 13 | Human cytomegalovirus | No |
| 5 | Influenza A virus | No | 14 | Rotavirus | No |
| 6 | Influenza B virus | No | 15 | Norovirus | No |
| 7 | Respiratory syncytial virus | No | 16 | Mumps virus | No |
| 8 | Adenovirus | No | 17 | Varicella-zoster virus | No |
| 9 | Rhinovirus | No | 18 | Mycoplasma pneumoniae | No |
1.2 Interfering substances: The following substances were added to the samples with specified concentrations, and their potential interference in the neutralization antibody test project of novel coronavirus (2019-nCoV) was evaluated. The results show that various interfering substances will not interfere with the detection results of this reagent.
| Interfering substances | Concentration | Interfering substances | Concentration |
| Bilirubin | ≤50mg/dL | Triglycerides | ≤15mmol/mL |
| Hemoglobin | ≤5g/L | Cholesterol L | ≤500mg/d |
| Rheumatoid factor | ≤500IU/mL | Human total IgG | ≤14mg/mL |
| Ribavirin | 0.4mg/mL | Fluticasone | 0.5mg/mL |
| Oxymetazoline | 10mg/mL | Dexamethasone | 5 mg/mL |
| Histamine hydrochloride | 10mg/mL | Triamcinolone acetonide | 5mg /mL |
| Tobramycin | 1mg/mL | Levofloxacin | 0.2 mg/mL |
| Oseltamivir | 1mg/mL | Azithromycin | 0.1 mg/mL |
| Zanamivir | 1mg/mL | Ceftriaxone | 0.4 mg/mL |
| Arbidol | 0.5mg/mL | Meropenem | 0.2 mg/mL |
2. Clinical research: Using the novel coronavirus (2019-nCoV)IgG antibody detection reagent (colloidal gold method) as the contrast reagent, 120 positive samples and 300 negative samples were selected for detection respectively, and the results are summarized as follows:
| 2019-nCoV IgG Ab detection reagent (colloidal goldmethod) | Sum | |||
| Positive | Negative | |||
 |
Positive | 115 | 5 | 120 |
| Negtive | 5 | 295 | 300 | |
| Sum | 120 | 300 | 420 | |
| Sensitivity | 95.83%, (95%CI: 90.62%~98.21%) | |||
| Specificity | 98.33%, (95%CI: 96.16%~99.29%) | |||
 |
Do not re-use |  |
Store at 2℃~30℃ |
 |
Consult instructions for use |  |
In vitro diagnostic medical device |
 |
Batch code |  |
Use-by date |
 |
Keep dry |  |
Keep away from sunlight |
 |
Authorized representative in the European Community |
 |
Manufacturer |

Please fill in your requirements, we will reply you as soon as possible.