



The kit consists of test card, sample buffer and swab.
Test card: It is composed of aluminum foil bag, desiccant, test strip and plastic card. Among them, the test strip is composed of absorbent paper, NC membrane, sample pad, binding pad and rubber sheet. The NC membrane T line (test line) is coated with 2019-nCoV Ab, the C line (quality control line) is coated with goat anti-mouse polyclonal Ab, and the binding pad contains a marker-labeled 2019-nCoV Ab.
Sample buffer: Phosphate buffer, surfactant, etc.
The kit is used for qualitative detection of 2019-nCoV Ag collected from human anterior nasal swab samples.It is for both home use and professional use.
As a new type, 2019-nCoV is a kind of β-CoVs. It can cause viral pneumonia, with main clinical manifestations of fever, fatigue and dry cough. A few patients are accompanied by nasal congestion, runny nose, sore throat, diarrhea and other symptoms. Critical cases often develop dyspnea and/or hypoxemia after a week, and those at serious condition rapidly progress to acute respiratory distress syndrome, septic shock, kidney failure and death.
This product adopts a lateral flow immunoassay method for the qualitative detection of 2019-nCoV Ag in anterior nasal swab samples from suspected patients. In the acute phase of infection, antigens are usually detected in anterior nasal swab samples. Positive result indicates the presence of viral antigens, but the clinical relevance of the patient's medical history and other diagnostic information is also necessary to determine the infection status. A positive result can not exclude bacterial infection or co-infection with other viruses.
The test results of this kit are for clinical reference only. It is recommended to conduct a comprehensive analysis of the condition based on the patient's clinical manifestations and other laboratory tests.
This kit is an immunoassay based on the principle of double antibody sandwich technology. As an indicator marker, the 2019-nCoV monoclonal antibody labelled with a marker is sprayed on the binding pad. During the test, the 2019-nCoV Ag in the sample is combined with the labelled 2019-nCoV monoclonal Ab to form an Ag-Ab complex. This complex migrates upwards on the membrane by capillary effect until it is captured by another 2019-nCoV monoclonal Ab pre-coated at the test line to form asandwich complex. If there is 2019-nCoV Ag in the sample, a red band will appear in the T area of the interpretation window. Otherwise, it is a negative result. The control line (C) is used for program control and should always be displayed if the test procedure is executed correctly.
(1) Collection method for anterior nasal swab: Insert the sampling swab into the nostril, and the swab tip should be inserted 2.5 cm from the edge of nostril. Roll the swab along the mucous membrane inside the nostril five times, and repeat the process with the same swab for the other nostril. (See Figure 1)
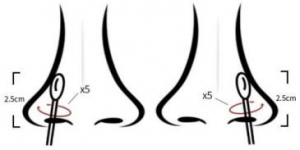
Figure 1 Collection method for anterior nasal swab
(2) Sample processing: The collected sample should be processed as soon as possible with the sample buffer provided by this kit (if it cannot be processed immediately, the sample should be stored in a dry, sterilized and strictly sealed container), stored for no more than 24h at 2℃~8℃, and kept for a long time at -70℃ (but avoid repeated freezing and thawing).
Read the instructions carefully before testing. Please return all reagents to room temperature before testing, and the test should be performed at room temperature. Washing hands before performing the test.
1. Sample processing (see Figure 2)
① Insert the sampled swab into the sample buffer, and rotate it close to the inner wall about 10 times to dissolve the sample in the solution as much as possible.
② Squeeze the swab tip along the inner wall of tube to keep the liquid flow into the tube as much as possible, remove and discard the swab.
③ Cover the dripper.

Figure 2 Sample processing

Figure 3 Detection procedure
① Take out the test card.
② Add 3 drops (about 80μL) of the processed sample extract to the loading well of test card, and then start the timer.
③ Read the results when the card is placed at room temperature for 15 minutes. The result is invalid after 20 minutes.
Interpretation of test card (Figure 4):
① Positive result: Both the quality control line (C line) and test line (T line) appears.
ADVICE:There is currently a suspicion of a COVID- 19 infection. Please contact a doctor / family doctor or the local health department immediately and have a PCR confirmatory test carried out. Please follow local guidelines for self-isolation.
② Negative result: Only the quality control line (C line) and no test line (T line) appears.
ADVICE: Please continue to comply with all applicable rules regarding contact with others and protective measures. Even if the test is negative, there may be an infection. If you suspect, repeat the test after 1-2 days, as the coronavirus cannot be accurately detected in all phases of an infection.
③ Invalid result: Quality control line (C line) fails to appear, so the test should be taken again.
ADVICE: This was possibly caused by an incorrect test execution. Please repeat the test. If the test results remain invalid, contact a doctor or a COVID- 19 test center.

Figure 4 Interpretation of test results

Please fill in your requirements, we will reply you as soon as possible.